Aromatic compounds
The cyclic organic compounds which contain at least one aromatic ring that resembles benzene are called aromatic compounds.
Example :
1) Benzene
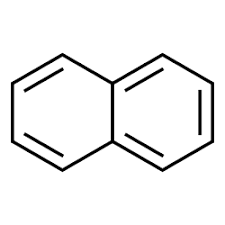
2) Naphthalene
Aryl group and Phenyl group
Aryl group :
Aromatic Hydrocarbons are called Arenes.
Aryl group is derived from arenes and represented as Ar.
Phenyl group :
C6H5 is called Phenyl group.
Benzene was originally called Phene and the Phenyl group is derived from benzene.
Characteristics of Aromatic compound
1) Aromatic compounds contain higher percentage of carbon than corresponding aliphatic hydrocarbons (alkanes, alkenes,alkynes)
2) Aromatic compounds are more stable than alkanes.
3) Aromatic compounds inspite of the presence of double bond readily undergoes substitution reactions.
4) Aromatic compounds due to higher percentage of carbon content, burn with sooty flame.
Some important aromatic compound
1) Benzene
12) Thiophene
13) Benzene sulphonic acid
14) Furan 15) Anisole
Resonating Structures of Benzene
Preparation method Of benzene
1) When acetylene is reacted with Red hot iron tube at 873 k, it undergoes cyclic polymerization, gives the formation of benzene.
2) When Sodium benzoate is reacted with sodium hydroxide undergoes decarboxylation it gives the formation of benzene.
3) When Phenol is reacted with zinc dust, it undergoes reduction and gives the formation of benzene.
Reactions of benzene
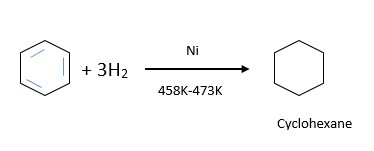
4) When Benzene is reacted with hydrogen in presence of Ni catalyst it gives the formation of Cyclohexane.
5) When Benzene is reacted with Ozone it gives the formation of Benzene triozonide which on hydrolysis with water in presence of zinc gives three molecules of Glyoxal.6) When Benzene is reacted with concentrated nitric acid in presence of concentrated H2SO4 it gives the formation of Nitrobenzene.
7) When Benzene is reacted with Sulfuric acid in presence of fuming H2SO4 it gives the formation of benzene sulphonic acid.
1) When Benzene is reacted with methyl chloride in presence of anhydrous AlCl3 it gives the formation of Toluene.Friedel-craft's alkylation reaction
2) When Benzene is reacted with ethyl chloride in presence of anhydrous AlCl3 it gives the formation of Ethyl benzene1)When Benzene is reacted with Acetyl chloride in presence of Anhydrous AlCl3 it gives the formation of Acetophenone
Friedel-craft's acylation reaction
Uses of aromatic compound
1) Aromatic compounds play important role in biochemistry of all living things.
2) Aromatic compounds are important in industry.
3) Toluene is used for glues,paints,thinners and nail-polish remover.
4) It is also used for printing process.
5) naphthalene used for pesticide.
6) Benzoic acid used as food preservatives.
7) Phenol is used in preparation of cosmetics.
Aromatic compounds are two types
1) Benzenoid and
2) Non-benzenoid compounds.






























No comments:
Post a Comment